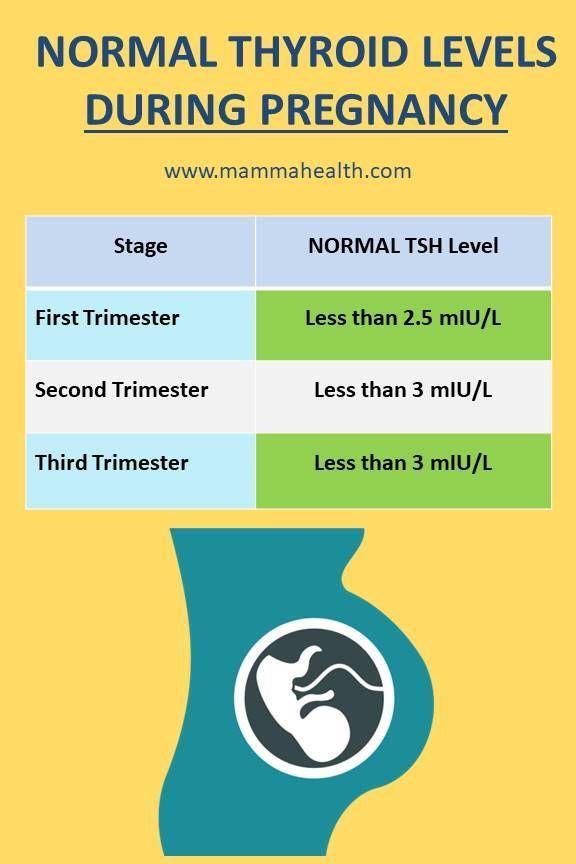
Normal Thyroid levels during pregnancy
Thyroid hormone is critical for brain development in the baby. Children born with congenital hypothyroidism (no thyroid function at birth) can have severe cognitive, neurological and developmental abnormalities if the condition is not recognized and treated promptly. With early treatment, these developmental abnormalities largely can be prevented. All newborn babies should be screened for congenital hypothyroidism so they can be treated with thyroid hormone replacement therapy as soon as possible.
Untreated severe hypothyroidism in the mother can lead to impaired brain development in the baby.
Recent studies have suggested that mild developmental brain abnormalities also may be present in children born to women who had mild untreated hypothyroidism during pregnancy. However, the ATA recommends checking a woman’s TSH as soon as pregnancy is confirmed in women at high risk for thyroid disease, such as those with prior treatment for hyper- or hypothyroidism, a family history of thyroid disease, a personal history of autoimmune disease, and those with a goiter.
Women with established hypothyroidism should have a TSH test as soon as pregnancy is confirmed. They also should immediately increase their levothyroxine dose, because thyroid hormone requirements increase during pregnancy. (See below for specific dosing recommendations.) If new onset hypothyroidism has been detected, the woman should be treated with levothyroxine to normalize her TSH values (see Hypothyroidism brochure).